-
A hospital experienced a large increase in the number of prostate cancer cases in the late 1980s after the implementation of the PSA blood test. Starting in 1992, when there were 200 new cases, the number of new cases began to decay exponentially. In 1993, there were 176 new cases. Estimate the year when there were about 136 new cases. (See Example 5.)
-
From the 1992 to 1993, the number of new cases dropped from 200 to 176. This is a decrease of
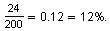
If the number of new cases decreased by the same percent each year, then the cases would have the following pattern.

The number of new cases should have dropped to about 136 in 1995.
Comments (0)These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.Showing 0 commentsSubscribe by email Subscribe by RSSThere are no comments. -
-
The number of lung cancer deaths at a hospital decreased from 50 in 2005 to 45 in 2006. Assuming the number of yearly deaths decays exponentially, estimate the year when there were about 30 deaths. (See Example 5.)
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
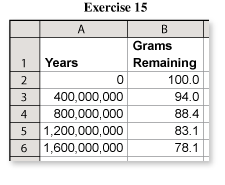
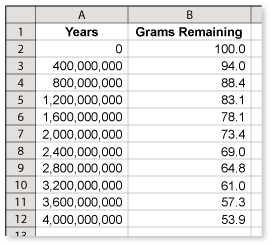
A sample of uranium ore originally contained about 100 grams of uranium-238. Use the spreadsheet to determine how long it will take the sample to decay to about 61 grams.(See Example 6.)
-
The easiest way to answer this question is to download the spreadsheet and extend the years.
From the spreadsheet, you can see that the number of grams is decreasing by 6% for each 400 million years. At this rate, it will take 3.2 billion years for the amount of uranium to decrease to 61 grams.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
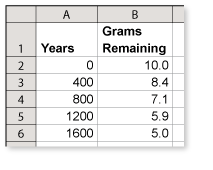
A scientist separates 10 grams of radium-226 from uranium ore and places it in a storage container. Use the spreadsheet to determine how long it will take the 10 grams of radium-226 to decay to about 3 grams. (See Example 6.)
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
Uranium-238 decays into thorium-234, which decays into protactinium-234m. This chain of decaying continues as shown in the diagram, which also includes radium-226 and radon-222.
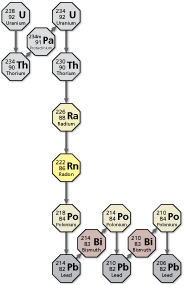
Consider all the radon gas produced by the decaying of radium in the storage container after 1200 years. (See Example 6.)
- Would all this radon gas still be in the storage container? Explain.
- Suppose a jar contains 50 grams of radon-222. About 34.7 grams of radon-222 remain after 2 days. Approximate the half-life of radon-222. Explain your reasoning.
-
- No, radon (Rn) itself has a half-life. So, some of the radon will have decayed into polonium.
-
After 2 days, the amount of radon-222 decayed to
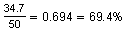
of the original amount. After 2 more days, the amount of radon-222 will be about

Because this is roughly half of the original amount, the half-life of radon-222 must be about 4 days.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
Radon itself is inert, so it is typically unreactive. However, the U.S. Environmental Protection Agency recommends that all homes be tested for radon. Why do you think elevated levels of radon gas in your home are a health hazard?
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.