-
Suppose you live near sea level (0 meters) and heat a pan of water until it boils.
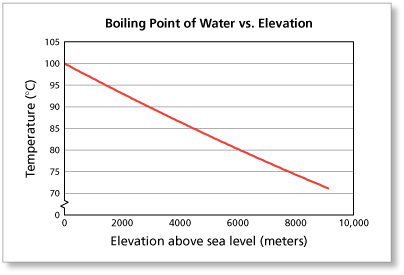
- At what temperature will you observe the water boiling?
- What might you infer about the boiling point of all water?
- Draw a set diagram that represents the inference from part (b).
-
- If you live near sea level, you should observe the boiling point of water to be about 100° Celsius.
- By repeatedly observing the boiling point of water to be 100° Celsius (at sea level), you might conclude that the boiling point of all water is 100° Celsius.
- One possible set diagram of this inductive reasoning is as follows.
Conclusion Based on Pattern
- All of the water I have observed at sea level boils at 100° Celsius.
- Therefore, all water at sea level boils at 100° Celsius.
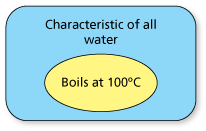
This is an example in which you can reach a false conclusion using inductive reasoning, because the reasoning was not based on a large enough or diverse enough sample.
Comments (0)These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.Showing 0 commentsSubscribe by email Subscribe by RSSThere are no comments. -
The elevation of La Paz, Bolivia is about 3600 meters above sea level.

- At what temperature will a resident of La Paz observe water boiling?
- What might the resident infer about the boiling point of all water?
- Draw a set diagram that represents the inference from part (b).
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
Write a syllogism that involves the boiling point of water at sea level (0 meters).

-
Here is one possible syllogism.
• Premise: If water at sea level is heated to a temperature of 100° Celsius, then it will boil. • Premise: Water at sea level is heated to a temperature of 100° Celsius. • Conclusion: The water boils. "From 1744 until 1954, 0°C was defined as the freezing point of water and 100°C was defined as the boiling point of water, both at a pressure of one standard atmosphere with mercury being the working material[citation needed] Although these defining correlations are commonly taught in schools today, by international agreement the unit "degree Celsius" and the Celsius scale are currently defined by two different points: absolute zero, and the triple point of VSMOW (specially prepared water). This definition also precisely relates the Celsius scale to the Kelvin scale, which defines the SI base unit of thermodynamic temperature (symbol: K)." (source: Wikipedia)
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
Write a syllogism that involves the boiling point of water in La Paz (about 3600 meters above sea level).

These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
Write a syllogism that involves the relationship between elevation and boiling point.

-
Here is one possible syllogism.
• Premise: If you move from sea level to a higher elevation, then the boiling point of water will be less than 100° Celsius. • Premise: You move from sea level to a higher elevation. • Conclusion: The boiling point of water is less than 100° Celsius.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
Is the logic in the statement valid? Draw a set diagram to analyze the argument.
If the elevation decreases, the boiling point increases. The boiling point increased, so the elevation must have decreased.

These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
As elevation increases, atmospheric pressure decreases. Write a syllogism that involves the relationship between atmospheric pressure and elevation.
-
Here is one possible syllogism.
• Premise: If you move from sea level to a higher elevation, then the atmospheric pressure decreases. • Premise: You move from sea level to a higher elevation. • Conclusion: The atmospheric pressure decreases.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
Suppose you perform the following experiment. You place water in a pressure cooker at an elevation of 5000 meters above sea level and adjust the pressure in the cooker to 1 atmosphere, which is the pressure at sea level (0 meters). When you heat the water, it boils at 100ºC. What can you conclude?
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.





