-
The figure shows light passing through a glass container that contains a substance. Absorbance is a unitless measure of the amount of light that a substance absorbs as light passes through it.
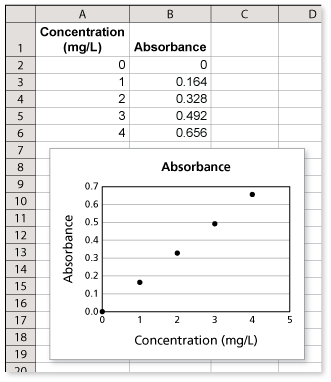
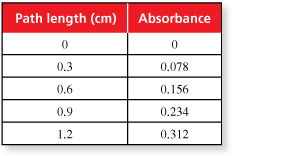
The table shows the absorbance of light with a wavelenght of 630 nanometers for a solution of the food dye Blue No.1 in a 1-centimeter glass container at various concentrations.
- Describe the pattern of the absorbance values
- Make a scatter plot of the data.
- Predict the next absorbance value in the pattern.
(See Example 5 and Example 6.)
-
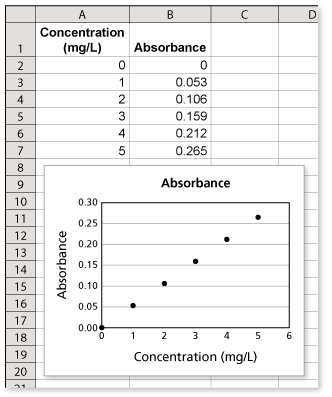
- Each time the concentration increases by 1 milligram per liter, the absorbance increases by 0.164.
- A scatter plot of the data is shown using a spreadsheet.
- The next absorbance value in the pattern would be for a concentration of 5 milligrams per liter. The absorbance for this concentration should be
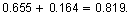
Comments (0)These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.Showing 0 commentsSubscribe by email Subscribe by RSSThere are no comments. -
The figure shows light passing through a glass container that contains a substance. Absorbance is a unitless measure of the amount of light that a substance absorbs as light passes through it.
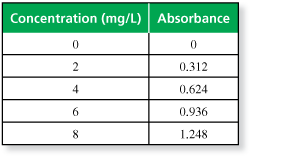
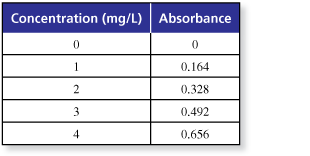
The table shows the absorbance of light with a wavelength of 625 nanometers for a solution of the food dye Green No. 3 in a 1-centimeter glass container at various concentrations.
- Describe the pattern of the absorbance values.
- Make a scatter plot of the data.
- Predict the next absorbance value in the pattern.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
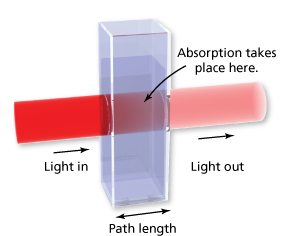
The figure shows light passing through a glass container that contains a substance. Absorbance is a unitless measure of the amount of light that a substance absorbs as light passes through it.

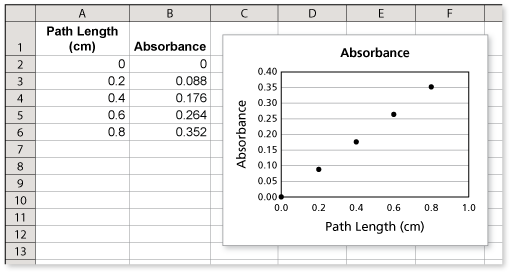
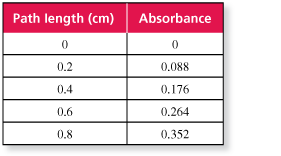
The table shows the absorbance of light with a wavelength of 527 nanometers for a solution of the food dye Red No. 3 with a concentration of 4 milligrams per liter in glass containers of various path lengths.
- Describe the pattern of the absorbance values.
- Make a scatter plot of the data.
- Predict the next absorbance value in the pattern.
(See Example 5 and Example 6.)
-
- Each time the path length increases by 0.2 centimeter, the absorbance increases by 0.088.
- A scatter plot of the data is shown using a spreadsheet.
-
The next absorbance value in the pattern would be for a path length of 1 centimeter. The absorbance for this path length should be
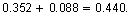
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
The figure shows light passing through a glass container that contains a substance. Absorbance is a unitless measure of the amount of light that a substance absorbs as light passes through it.

The table shows the absorbance of light with a wavelength of 500 nanometers for a solution of the food dye Red No. 40 with a concentration of 5 milligrams per liter in glass containers of various path lengths.
- Describe the pattern of the absorbance values.
- Make a scatter plot of the data.
- Predict the next absorbance value in the pattern.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
The figure shows light passing through a glass container that contains a substance. Absorbance is a unitless measure of the amount of light that a substance absorbs as light passes through it.
The absorbance of light with a wavelength of 428 nanometers for a solution of the food dye Yellow No. 5 in a 1-centimeter glass container is proportional to the concentration of Yellow No. 5. The absorbance of a solution with a concentration of 2 milligrams per liter is 0.106. What is the absorbance of a solution with a concentration of 5 milligrams per liter? (See Example 5 and Example 6.)
-
You are given that the absorbance is proportional to the concentration. You also know that
- when the concentration is 0, the absorbance is 0, and
- when the concentraiton is 2, the absorbance is 0.106.
This is enough information to determine that the absorbance increases by 0.053 each time the concentration increases by 1 milligram per liter. The spreadsheet shows that when the concentration is 5 milligrams per liter, the absorbance is 0.265.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
The figure shows light passing through a glass container that contains a substance. Absorbance is a unitless measure of the amount of light that a substance absorbs as light passes through it.

The absorbance of light with a wavelength of 484 nanometers for a solution of the food dye Yellow No. 6 with a concentration of 6 milligrams per liter is proportional to the path length. The absorbance of the solution in a 0.5-centimeter glass container is 0.162. What is the absorbance of the solution in a 1.1-centimeter glass container? (See Example 5 and Example 6.)
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.