-
Subjects in a study receive daily doses of a medicine for 10 weeks. In the first week, they receive 100 milligrams each day. Each week thereafter, the dosage decreases 10% from the previous week. Write a formula that represents the daily dosage during each week.
-
The formula for this exponential decay is
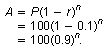
P = 100 people, r = 10%, n is the number of weeks
Comments (0)These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.Showing 0 commentsSubscribe by email Subscribe by RSSThere are no comments. -
-
Subjects in a study receive daily dose of a medicine for 10 weeks. In the first week, they receive 100 milligrams each day. Each week thereafter, the dosage decreases 10% from the previous week. Make a table showing the daily dosage during the 10 weeks.
A spreadsheet is available to help you complete this exercise.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
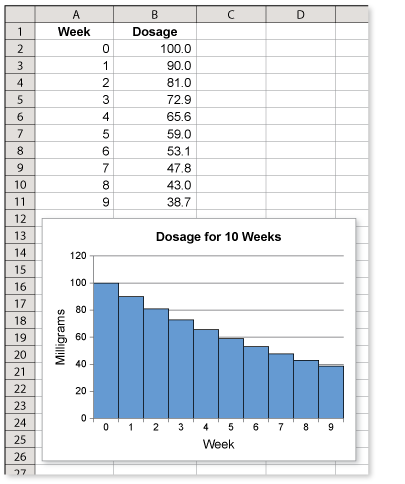
Subjects in a study receive daily doses of a medicine for 10 weeks. In the first week, they receive 100 milligrams each day. Each week thereafter, the dosage decreases 10% from the previous week. Sketch a graph showing the decrease in the daily dosage during the 10 weeks.
A spreadsheet is available to help you complete this exercise.
-
The formula for this exponential decay is
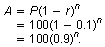
P = 100 people, r = 10%, n is the number of weeks
Use a spreadsheet to graph the dosage for the 10 weeks.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
Subjects in a study receive daily doses of a medicine for 10 weeks. In the first week, they receive 100 milligrams each day. Each week thereafter, the dosage decreases 10% from the previous week. The half-life of the medicine is 24 hours. How much of the medicine received in a dose during week 10 remains in the patient's bloodstream after 24 hours?
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
Most technetium is man-made in nuclear reactors. There are many isotopes of technetium, two of which are technetium-99 and technetium-99m. The half-life of technetium-99 is 210,000 years. How much of a 120-gram sample of technetium-99 will remain after 630,000 years?
-
After 210,000 years,
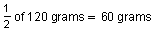
remain. After another 210,000 years
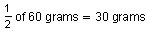
remain. After another 210,000 years
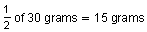
remain. So, after 630,000 years, 15 grams remain.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
Most technetium is man-made in nuclear reactors. There are many isotopes of technetium, two of which are technetium-99 and technetium-99m. The half-life of technetium-99 is 210,000 years. A vial contains 10 grams of technetium-99m. Use the spreadsheet to determine how long it will take the 10 grams of technetium-99m to decay to about 2.1 grams
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
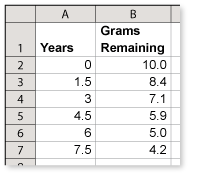
A vial contains 10 grams of technetium-99m. The table shows the amount remaining after various periods of time.
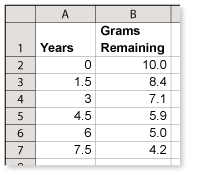
What is the half-life of technetium-99m? Explain your reasoning.
-
From the table, you can see that it took 6 years for the amount to decay from 10 grams to 5 grams.
This implies that the half-life is 6 years.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use. -
-
Most technetium is man-made in nuclear reactors. There are many isotopes of technetium, two of which are technetium-99 and technetium-99m. The half-life of technetium-99 is 210,000 years. A solution containing technetium-99m is often injected into a patient to help diagnose problems in the body. Explain why a hospital or veterinary clinic should not order excessive amounts of technetium-99m with the intent of placing it in storage.
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.