-
Determining the age of ancient fossils or artifacts once was the job of paleontologists. By comparing the placement of an object with the age of the rock and silt layers in which it was found, scientists could usually make a general estimate of the object's age. This method is rather limited because many objects are found in areas whose ages are not known, such as a cave or frozen in ice.
In 1907, American chemist Bertram Boltwood (1870-1927) proposed that rocks containing radioactive uranium could be dated by measuring the amount of lead in the rock. (When uranium decays, it changes into lead over a long period of time.) So, the greater the amount of lead, the older the rock. This method is also limited because it only applies to objects containing uranium. The benefit of this discovery, however, was it showed that radioactive dating was possible.
The first method for dating organic objects, such as the remains of plants and animals, was developed by American chemist, Willard Libby (1908-1980). Libby began testing his carbon-14 dating procedure by dating objects whose ages were already known. He found that his methods, while not as accurate as he had hoped, were fairly reliable. He continued his research and was eventually able to determine the age of an object up to 50,000 years old with a precision of plus-or-minus 10%. (The accuracy of the estimate of the age depends upon assumptions concerning the past intensity of cosmic radiation, accuracy of the equipment used, and other factors.)
Scientists have developed other dating methods, including the uranium-thorium method, the potassium-argon method, and the rubidium-strontium method, all of which are based on the transformation of one element into another. (Source: Encyclopedia.com)
-
National Fossil Day began on October 13, 2010 as part of Earth Science Week. If you'd like to find a fossil park or ancient history museum near you, visit the National Park Service.
-
Use the formula for exponential decay using half-life.
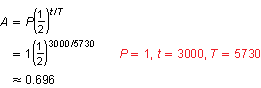
So, the ratio of carbon-14 to carbon-12 in the soil samples was about 0.696.
-
Comments (1)
These comments are not screened before publication. Constructive debate about the information on this page is welcome, but personal attacks are not. Please do not post comments that are commercial in nature or that violate copyright. Comments that we regard as obscene, defamatory, or intended to incite violence will be removed. If you find a comment offensive, you may flag it.
When posting a comment, you agree to our Terms of Use.Showing 1 commentsSubscribe by email Subscribe by RSSRon Larson (author)1 decade ago |The plot of "Jurassic Park" is that scientists will be able to use DNA from extinct animals to recreate them. I think this is a fascinating idea.0 0





